1. Introduction
Molecular or Atomic aggregates among which one dimension within 1 - 100 nm range are referred to as NPs. Research has demonstrated the significance of NPs for enhancing human health, electrical, magnetic, as well as optoelectronic, biological, pharmacological, cosmetic, energy, environmental, catalytic, as well as material uses
. The material's characteristics of NPs differ dramatically from those at larger scales, at the nano scale, quantum phenomena have an impact on the characteristics and behaviour of particles. NP interacts with other naturally occurring molecules differently than a molecule composed of the same chemical components at the microscale because it has such a huge surface area per unit mass
| [2] | Prathna T C, Chandrasekaran N, Raichur A M, Mukherjee A: Kinetic Evolution Studies of Silver Nanoparticles in a Bio based Green Synthesis Process, Colloids Surf. A, 377 (2011) 212–216. https://doi.org/10.1016/j.colsurfa.2010.12.047 |
[2]
.
The semiconducting material Cadmium Sulfide (CdS) is a belonging to the II – VI group with a direct band gap of 2.42 eV in the bulk state at ambient temperature. It has three distinct crystal structures: cubic zinc blend, hexagonal wurtzite, as well as high pressure rock salt phases. The quantum confinement effect is the cause for nanosize materials to exhibit distinct variations in their optical, electrical attributes, melting point, transition temperature, further crystallanity compared to their bulk counterparts. These variations are primarily influenced by the scale of the crystallites. Because of its excellent absorption coefficient, CdS NPs have promising advantages such as photovoltaic devices, light emitting diodes (LEDs)
, photo detectors
| [4] | Wang Y, Ramanathan S, Fan Q, Yan F, Morkoe H, Bandyopadhyay R S: Electric Field Modulation of Infrared Absorption at Room Temperature in Electro Chemically Self Assembled Quantum Dots, J. Nano-sci. Nanotechnol., 6 (2006) 2077-2080. https://doi.org/10.1166/jnn.2006.359 |
[4]
, electric driven lasers
, photocatalysts
, optoelectronics, heterojunction solar cells
| [7] | Oladeji I O, Chow L, Ferekides C S, Viswanathan V, Zhao Z: Metal/CdTe/Cds/Cd1-xZnxS/TCO/Glass: A New CdTe Thin Film Solar Cell Structure, Sol. Energy Mater. Sol. Cells, 61 (2000) 203-211. https://doi.org/10.1016/S0927-0248(99)00114-2 |
[7]
. CdS NPs could be altered with B, In, Ga, and Al to achieve n-type conductivity, and CdS material can indeed be modified with Cu, Au, along with Ag to get p-type conductivity
.
Synthesis approaches of nanomaterials were classified into two categories, one is traditional synthesis and the other is Green Synthesis methods. Traditional synthesis methods comprehend chemical precipitation, chemical reduction, chemical displacement reaction and template based chemical route. The different methods followed by the researchers to synthesize CdS NPs are chemical precipitation approach
| [9] | Singh V, Chauhan P: Structural and Optical Characterization of CdS Nanoparticles Prepared by Chemical Precipitation Method. Journal of Physics and Chemistry of Solids, 70 (2009) 1074–1079. https://doi.org/10.1016/j.jpcs.2009.05.024 |
[9]
, Solvo thermal procedure
, Laser ablation process
| [11] | Lalayan A, Avetisyan A, Djotyan A: The Luminescence Properties of the Colloidal GaAs and CdS Semiconductor Quantum Dots, Laser Phys. Lett. 2 (2005) 12-15.
https://doi.org/10.1002/lapl.200410138 |
[11]
, Hydrothermal process
, Photo chemical method
, Microwave heating procedure
| [14] | Wada Y, Kuramoto H, Anand J, Tikamura T, Sakata T, Mori H, Yangida S: Microwave-Assisted Size Control of CdSNanocrystallites, J. Mater. Chem., 11 (2001) 1936-1940.
https://doi.org/10.1039/B101358K |
[14]
, Photo etching
| [15] | Torimoto T, Reyes J P, Iwasaki K, Pal B, Shibayama T, Sugawara K, Takahashi H, Ohtani B: Preparation of Novel Silica-Cadmium Sulfide Composite Nanoparticles Having Adjustable Void Space by Size-Selective Photoetching, J Am Chem Soc. 125 (2003) 316-317.
https://doi.org/10.1021/ja0278133 |
[15]
, and Ultrasonic irradiation method
| [16] | Wang G Z, Chen W, Liang C H, Wang Y W, Meng G W, Zhang L D: Preparation and Characterization of CdS Nanoparticles by Ultrasonic Irradiation, Inorgonic Chemistry Communications, 4 (2001) 208-210.
https://doi.org/10.1016/S1387-7003(01)00172-1 |
[16]
. CdS NPs with improved optical properties are being produced using a chemical precipitation technique, and their photoluminescence (PL) behavior was investigated. The results show that the PL peaks became more intense when the capping reagent concentration is raised and also explained why PL intensity dips as annealing temperature rises
. Chemical displacement technique is used to produce CdS NPs, and they discovered that the NPs have a relative dielectric constant that is noticeably greater than that of the bulk CdS powder
, Green Synthesis of Cadmium Oxide NPs using syzygiumcumini
| [19] | SivakumarSivalingam, JyotiKavirajwar, K. Seethalakshmi, JayagopiGayathri and A.Roniboss. Green synthesis of cadmium oxide nanoparticles (CdO-NPS) using syzygiumcumini: exploring industrial applications of CdO NPs as a corrosion inhibitor of mildsteel in the acidic environment. RSC Adv., 2024, 14, 7932-7939. https://doi.org/10.1039/d4ra00560k |
[19]
, Green synthesis of copper oxide NPs using Ficusracemosa leaf extract and investigation of its antibacterial properties
| [20] | Maurya, A. K., Varshney, S., Upadhyay, S., Gupta, A., Jeyakumar, E., Lawrence, R., &Mishra, N. Green synthesis of copper oxide nanoparticles using Ficusracemosa leafextract and investigation of its antibacterial properties. Advances in Materials andProcessing Technologies, ((2024) 1–21.
https://doi.org/10.1080/2374068X.2024.2402970 |
[20]
, Facile Green Synthesis of CdS Nanoparticles in a Glycine Medium for Waste Valorization of Ni-Cd Battery
| [21] | Azmand, A., Firoozi, S. &Haghshenas, D. F. Facile Green Synthesis of CdS Nanoparticles in a Glycine Medium for Waste Valorization of Ni-Cd Battery. Waste Biomass Valor 16, 5939–5951 2025. https://doi.org/10.1007/s12649-025-03026-4 |
[21]
, Synthesis of Silver and Copper oxide nanoparticles using Ficusracemosa leaf extract
| [22] | Akhilesh Kumar Maurya, ShagunVarshney, Vinod Verma, Hifzur R., Siddique, Nidhi Mishra. Synthesis of Silver and Copper oxide nanoparticles using Ficusracemosaleaf extract: characterization, anticancer potential, and dye degradation efficacy. Journal of Umm Al-Qura University for Applied Sciences. 2025, 11, 581–594. https://doi.org/10.1007/s43994-024-00182-6 |
[22]
. Much priority given to CdS NPs due to some encouraging factors like, the availability of discrete energy levels, variable bandgap, dimensional dependent optical properties, good chemical stability, convenient including cost effective preparation techniques.
The literature could not find the preparation of CdS NPs employing Aolevera as a stabilizing or reducing agent. The comparison of three different cadmium precursors such as cadmium chloride, cadmium nitrate and cadmium sulphate with Aloe vera leaf gel is important, because of the interaction of cadmium precursor with Aloe vera effects differently and lead to changes in optical property such as tunable band gap, physico-chemical properties, size formation, morphology and fluorescence. The motivation for applying green synthesized CdS NPs as photocatalysts in Photocatalitic and PL studies is that these CdS NPs exhibit the structural and optical properties and hence influencing the efficiency of photo catalysts in degrading organic pollutants or dyes.
The photocatalytic activity as well as photo luminescence studies carried out for natural stabilizer Aloe vera leaf gel extract has been applied as catalyst due to the deterioration of two dyes which are Methylene Blue (MB) as well as Rhodamine B (Rh B) to the exposure of visible light and studied their catalytic activity for three hours in which first one hour is in dark observation and continued two hours manifestation under visible light. The luminescence studies of as-prepared CdS NPs stabilized by Aloe vera leaf gel extract are explained in the present work. All the Figures in this chapter designated as (a) indicates the CdS NPs prepared using cadmium chloride, (b) signifies CdS NPs produced from cadmium nitrate and (c) demonstrates the CdS NPs composed out of cadmium sulphate precursors.
CdS NPs prepared using laser liquid ablation (LLA) in a natural Aloe vera medium and found particles size of about 40 nm in the literature
| [23] | RashaShakir Mahmood, DhiaHadi Hussain, Nibras Abdul Ameer Aboud. Green laser-ablated CdS nanoparticles in aloe vera medium: structural, optical, electrochemical, andsynergistic photocatalytic–adsorptive performance for pentachlorophenol degradation. Journal of Materials Science: Materials in Electronics. 2025, 36: 2135.
https://doi.org/10.1007/s10854-025-16253-1 |
[23]
.The novelty of using Aloe vera in preparing CdS NPs in the present work is that various cadmium precursors such as cadmium chloride, cadmium nitrate and cadmium sulphate are used as a source of cadmium and obtained NPs size of 3 nm with enhanced optical band gap at room temperature ambience with cost effective method and testing CdS NPs against Methylene Blue (MB) and Rhodamine B (RhB) dyes. Aloe vera assisted CdS NPs could reduce the toxicity of CdS due to the phytochemicals present in Aloe vera, tunable band gap CdS NPs have applications in treating the waste water, luminescence, hetero junction solar cells.
2. Experimental
The sodium sulphide (Na2S.H2O) solution of 0.1M was prepared by adding 0.7804 g in aqua solution of 100 ml Millipore water, then the freshly prepared Aloe vera leaf gel extract (30 weight percent of Cd source i.e., 36 ml for Cd(NO3)2, 62 ml for CdSO4, and 30 weight percent of Cd source for CdCl2 i.e., 55 ml) is added to the above solution slowly drop by drop while stirring. The cadmium precursor solution (0.05M of CdCl2, 0.05M of Cd(NO3)2, and 0.1M CdSO4 separately added in 100 ml Millipore water) added to the above mixture, 1M of NaOH was added to the solution obtained above and adjusted the pH appraisal to 11. The above said solution underwent stirring at 900 rpm for 4 hrs continuously. Finally an orange or yellow color solution has formed.
In synthesis process without adding any NaOH solution to the mixed solution of cadmium precursor and aloe vera extract, without adjustment pH value reached 11 for cadmium chloride precursor, 6 for cadmium sulphate precursor and got good XRD graphs at these values, and after several repetitions optimized pH value as 11 for cadmium nitrate precursor.
The final precipitate was washed several times and dried it to get in powder form. The final product is in yellow color and this material was characterized by utilizing the X-ray Diffraction and discovered to have CdS NPs. UV Visible absorption spectroscopy, Fourier Transform Infrared spectroscopy and Scanning Electron Microscopy and Small Angle X-ray Scattering techniques utilised to determine the band gap along with functional groupings, morphology as well as particle size of as prepared powder.
The XRD spectrums abide inscribed employing the inel equinox 3000 X-ray diffractometer at 40 kV and 25 mA with Cuk
α radiation wavelength of 1.540560 Å obsessed among position sensitive detector. The material's optical characteristic was recognized by utilizing the UV-Visible spectris 2092 spectrophotometer. The SHIMADZU FTIR-8400S spectrometer was used to record the FTIR spectrum in the 400–4500 cm
-1 range. Using a scanning electron microscope (ZEISS Special Edition 18), the morphology of the CdS NPs was determined. A dual energy Mo-Cr-SAXS laboratory system (Model Xeuss from M/s Xenocs, France) was used to perform the SAXS measurements
. To extract the size, IRENA macros were utilised to model fit the data and volume distribution of the CdS NPs
. The nanoparticles' shape and average particle size were verified by the use of a Transmission Electron Microscope (TEM) type Philips CM 20 that operated at 200kV. JobinYvon Fluorolog-3-11 spectroflurimeter was employed to get the photoluminescence properties of CdS NPs. The excitation was obtained from a 450W Xenon lamp source (Oriel, model 9119), the photons were counted by a detector PMT as well as the data were analyzed by DATA MAX/GRAMS/31 software.
Under 300 W of tungsten light, two dyes, Methylene Blue (MB) and Rhodamine B (RhB), with starting engrossment of 0.5×10-5mol/L, were decomposed to see how good the CdS NPs worked as photocatalysts. The catalyst concentration used for the dye degradation is 50 mg/100 ml of the dye solution of MB and RhB. Before irradiation, the mixture was stirred for one hr in the dark to reach equilibrium between adsorption and/or desorption. After that, the lamp was turned on, and for every 30 min, a sample of about 3 mL was taken to measure the photocatalytic degradation. Before measurements, the photocatalyst was taken out of the solution by spinning the sample in a centrifuge. All of the photocatalytic tests were done twice to make sure that the results are correct.
3. Results and Discussion
3.1. Characterization of Powder
Figure 1 (a-c) presents XRD spectra of Aloe vera gel extract encapsulated CdS NPs. The XRD peaks located at 2θ positions of 26.38
o, 29.84
o, 44.42
o, 51.96
o and 71.14
o are commenced to reflect (111), (200), (220), (311) and (331) planes, accordingly. These peaks matched well with the cubic cadmium sulphide (JCPDS Card No.89-0440). Apart from these planes, there are additional intensity peaks present in
Figure 1 (a) at 2θ values of around 45.44°,75.23°which could be indexed to scattering from (107) including (211) planes of cadmium chloride (JCPDS Card No.89-1568) and this may be owing to presence of small residues of cadmium chloride because of its incomplete dissolve.
The peak at 2θ value of 56.43°endows to reflectfrom (222) plane of sodium chloride (JCPDS Card No.89-3615) is due to reaction of cadmium chloride along with sodium sulfide which employed during reaction. The average size of the crystallites found as 8 nm for
Figure 1(a), 3 nm for 1 (b) and 3 nm for 1 (c). The lattice parameter reckoned from XRD information is 5.7917 Åwhich is very nearer to that of the reported value 5.8304 Å with a disparity of 0.0387 Å. The broadness of the peaks in the XRD spectra is the witness for the nano size particles among three graphs in XRD spectra.
Figure 1. XRD spectrums of Aloe vera extract assisted CdS NPs produced by using: (a) cadmium chloride, (b) cadmium nitrate including (c) cadmium sulphate precursors.
The broadness is larger for the CdS NPs prepared from the cadmium nitrate precursor comparing to other two cadmium sulphate and cadmium chloride salts. The CdS NPs prepared from cadmium chloride precursor exhibiting extra peaks other than the CdS phase is may be due to that the less effect of Aloe vera leaf gel stabilizer on formation of CdS NPs.
Figure 1 (a) confirming that there exists two sorts of powders existing one is in nano range along with the secondary peaks consent coarse-grained powder and latency of this is very small in CdS NPs prepared from cadmium nitrate precursor shown in
Figure 1 (b).
3.2. Studies of Surface Morphology Along with Elemental Analysis
SEM micrographs of Aloe vera gel stabilized CdS NPs prepared from various cadmium salts are shown in
Figure 2 (a-c). Since CdS NPs are seen to be somewhat aggregated in SEM images, it can be the result of a higher pH value during synthesis. Morphology of as prepared CdS NPs is both spherical, facet shaped particles which can be witnessed in
Figure 2 (a) and the CdS NPs made up of extremely minute particles and having spherical shape unveil in
Figure 2 (b).
Along with spherical morphology some irregular shaped NPs present as flocks depicting dense aggregation of crystallites in remaining cases shown in
Figure 2 (c). All samples from
Figure 2 (d-f) of EDS spectra reflect the existence of Cd and S elements clearly which confirms that nano particles are made up of Cd and S. The proportions of carbon, calcium, oxygen, sodium, and chlorine in the EDS spectra of CdS NPs validate the presence of phyto- chemicals of Aloe vera leaf gel stabilizer
. Analyzing EDS Spectra is also helpful in comprehending the Stoichiometric ratio of the cadmium to sulfur ratio. The CdS NPs derived from cadmium chloride as well as cadmium nitrate have cadmium to sulfur ratio of about 1:1, while the CdS NPs generated from cadmium sulfate have cadmium to sulfur ratio of nearly 1:2.
Figure 2. SEM images (a-c) and EDS spectrums (d-f) of Aloe vera leaf gel capped CdS NPs extracted from: (a, d) cadmium chloride, (b, e) cadmium nitrate along with (c, f) cadmium sulphate precursors.
With the help of TEM, the size besides morphology of the NPs is examined. In TEM images of Aloe vera leaves gel stabilized CdS NPs processed from discrepant cadmium precursors, including cadmium chloride, cadmium nitrate, in addition to cadmium sulphate shown in
Figure 3 (a-c), numerous spherical NPs are visible. Using Image J software, more than 250 and up to 5000 particles are measured, with the majority of the observed particles with a maximum dimension of under 10 nm. Employing cadmium chloride, cadmium nitrate, further more cadmium sulphate as starting materials, Aloe vera gel stabilized CdS NPs are found to have average particle sizes of 2.61, 2.51, along with 2.62 nm, respectively. The high sample concentration that is put onto the grid and the length of the probe sonication that is used to dissolve the powdered sample into solution are both potential causes of aggregate formation
| [28] | Rao M D, Pennathura G: Green Synthesis and Characterization of Cadmium SulphideNanopart- icles from ChlamydomonasReinhardtii and their Application as Photo- catalysts. MRB (Materials Research Bulletin) 8929 (2016) 1-44. |
[28]
.
The SAED images of Aloe vera leaf gel stabilized CdS NPs are analyzed and clearly demonstrate the CdS cubic structure. The presence of smaller particles is confirmed by the intense rings in an electron diffraction image depicted in
Figure 3 (b) in place of the normal dots. The d spacings from the JCPDF data of the CdS cubic crystalline phase are compatible with the d spacings evaluated for all significant rings in these samples and the results are in greater conformity with the obtained from XRD and SAXS analyses.
Figure 3. TEM micrographs, size distribution of Aloe vera leaves gel stabilized CdS NPs prepared from: (a) cadmium chloride, (b) cadmium nitrate including (c) cadmium sulphate.
The more broadness of peaks in XRD spectra, spherical morphology of small nano size particles also confirmed from TEM for the Aloe vera gel stabilized CdS NPs prepared from cadmium nitrate precursor.
3.3. Micro Structural Analysis
The quantitative micro-structural analysis is done by SAXS characterization.
Figure 4 shows the SAXS intensity profiles and volume distribution curves of as-synthesized Aloe vera leaf gel extract stabilized by Cadmium precursors which consist of cadmium chloride, cadmium nitrate along with cadmium sulphate, are the sources of CdS NPs. Substantial scattering through chemical heterogeneity, specifically CdS NPs at the midrange q-regions (Guinier Region) between 0.3 to 1 nm
-1 is seen in SAXS profiles. CdS NPs stabilized with Aloe vera leaf gel extract exhibits bimodal size distribution. The bimodal size distribution indicates the presence of two different sizes based on the band bap values of NPs present in the prepared powder which reflects two maxima in the volume distribution curves of
Figure 4.
The dispersed NPs with an average size of nearly equal volume of fractions 3.8 nm small in number and large number of particles having distribution with in 2 nm range are depicted in the CdS NPs derived from cadmium chloride, while the dispersion of cadmium nitrate is shown with an average size of massive volume fractions of 5.8 nm, along with a minimal volume fraction of closely 12 nm.
Figure 4. SAXS patterns and volume distribution curves of Aloe vera gel extract stabilized CdS NPs obtained by: (a) cadmium chloride, (b) cadmium nitrate including (c) cadmium sulphate precursors.
The CdS NPs produced out of cadmium sulphate provides the distribution's with average size of small volume fraction of 3.4 nm, large volume fraction of 9.4 nm, along with medium volume fraction of 6 nm. The primary allocation is the predominant one and having this size distribution by large number of particles that is approximately 2, 5 and 3 nm for the CdS NPs produced from cadmium chloride, cadmium nitrate including cadmium sulphate.
3.4. Studies of Functional Groups
The FTIR graphs of as synthesized CdS NPs stabilized by Aloe vera leaf gel extract is shown in
Figure 5 (a-c) which are composed with various cadmium sources like cadmium chloride, cadmium nitrate including cadmium sulphate and
Figure 5 (d) demonstrates FTIR curve of Aloe vera leaf gel stabilizer. The broad peak found in the high energy region of FTIR peak at 3451 cm
-1 gives O-H stretch vibrations of water molecules. The small intense broad peak in the range 2088 cm
-1 is ascribed to anti-symmetric as well as symmetric vibrations of the –CH2 groups of the hydrocarbons or bending vibrations of water molecule.
The strong intense narrow sharp peak at 1631 cm
-1 is assigned to C=C stretching. The vibration of the methanol is noticed at 1383 cm
-1 because it is employed in the procedure, this peak is very small and can be attributed to CH
3 bending deformation
| [29] | Kamble M M, Rondiya S R, Bade B R et al.: Optical, Structural and Morphological Study of CdS Nanoparticles: Role of Sulfur Source, Nanomaterials and Energy, 9 (2020) 72-81.
https://doi.org/10.1680/jnaen.19.00041 |
| [30] | Shruti G, Kumar T K: Catalytic Reduction of Organic Dyes with Green Synthesized Silver Nanoparticles Using Aloe vera Leaf Extract, Journal of Nanoscience, Nano Engineering and Applications, 9 (2019) 1-13.
https://doi.org/10.37591/jonsnea.v9i2.661 |
[29, 30]
or S=O group
| [31] | Kulkarni P, Abhijit N, Omkar K, Rahul S, Dhanshri L, Pooja K: Phytochemical and FTIR Analysis of TinisporaCordfilia, International Journal of Current Research, 10(2018) 75331-75334. https://doi.org/10.24941/ijcr.33147.11.2018 |
[31]
and the small sharp peak near to 1110 cm
-1 is ascribed to C-H bending vibrations. The IR peaks observed in the spectrum ranging at around 570 cm
-1 is because of Cd-S stretching
.
Table 1 displays a number of absorption peaks that can be connected to several functional groupings.
Table 1. The appropriate functional categories of CdS NPs capped by an Aloe vera leaves gel stabilizer and their wave numbers.
Sl.No. | Wave Number (cm-1) | Bonding of Chemical Compounds |
1 | 3451 | –OH stretching of water molecule |
2 | 2088 | Anti symmetric as well as symmetric vibrations of the –CH2 groups of hydro carbons |
3 | 1631 | Assigned to C=C stretching |
4 | 1383 | CH3 bending deformation or sulfate S=O group |
5 | 1110 | Attributed to C-H bending vibrations |
6 | 570 | Cd-S stretching |
Figure 5. FTIR spectra of Aloe vera leaf gel extract stabilized CdS NPs concocted out of: (a). cadmium chloride, (b) cadmium nitrate, (c) cadmium sulphate including the (d) Infrared spectrum of Aloe vera leaf gel extract.
3.5. Optical Studies
Figure 6 (a-c) depicts the UV-Visible absorbance characteristics of Aloe vera leaf gel extract capped CdS NPs braced out of cadmium chloride, cadmium nitrate together with cadmium sulphate as well as
Figure 6 (d-f) manifests the plots of photon energy vs. the square of the product of absorption coefficient, energy. The wavelength of maximum absorption around 215, 224 in addition to 254 nm specify the substance's inherent surfactant content
| [33] | Moghaddasi S M, Kumar V S: Aloe vera their Chemicals Composition and Applications: A Review, Int J Biol Med Res. 12 (2011) 466-471. |
[33]
.
The CdS NPs prepared out of cadmium nitrate is found to be having large band gap of 5.18 eV furthermore absorbance as compared to that of the CdS NPs prepared by using cadmium chloride is exhibiting band gap of 5.04 eV in addition to cadmium sulphate which is having band gap between 3.58 and 5.09 eV. This finding indicates that the absorption edge's blue shift, that means the absorption wavelength shifted to lower wavelength side causes wider band gap is occurred in all the materials.
Figure 6. Absorption spectra and band gap energy plots of Aloe vera gel stabilized CdS NPs attained from cadmium chloride (a, d), cadmium nitrate (b, e) including cadmium sulphate (c, f) metal precursors.
The reduction in size of CdS NPs is because of the quantum confinement effect. The property of tunable band gap of CdS NPs posses substantial progress over the visible region which gives hope for the electronic, optoelectronic, semi conductor devices and gas sensors applications
| [34] | Dwivedi D, Dayashankar K, Dubey M: Synthesis, Structural and Optical Characterization of CdS Nanoparticles, Journal of Ovonic Research, 6 (2010) 57-62. |
[34]
.
3.6. Catalytic Study of Aloe Vera Leaves Gel Extract Mediated CdS NPs
Exemplary UV-Visible Absorption spectrum changes undergoing the solution of dye in terms of reaction duration is illustrated by
Figure 7 (a-c) to explain the changes in molecular properties of MB as a result of photocatalytic degradation. It can be seen from this Figure that, the original MB solution absorption spectrum has two peaks in the visible spectrum, featuring the greatest sensitivity at approximately 664 nm. Disintegration of bands causes the absorbance values to decrease during photo deterioration. The measured parameter for the process of photo catalytic decomposition has been determined to be the typical absorption of MB at around 664 nm. The relationship between MB concentration as well as absorbance is proportional.
The insignificance of the downstream of MB as well as RhB dyes in the dark and in the existence of CdS may be observed in
Figures 7 and 8 [35]. The down fall in maximum absorption and lost its peak wavelength value observed for the Aloe vera included CdS NPs obtained from cadmium chloride, cadmium nitrate along with cadmium sulphate being 12, 12 and 47% of its MB dye, respectively after 180 min. Aloe vera stabilized CdS NPs synthesized from cadmium sulphate is exhibiting the highest activity i.e., 47% which is higher than the reported value of 30.55% photocatalytic efficiency observed for the Hibiscussrosa-sinesis leaves extract capped CdS NPs for degradation of Methylene Blue Dye
| [36] | Salian A. R, Henriques S. K, Ardra S. S. Synthesis of Cadmium Sulfide Nanoparticlesvia Green Route for Photocatalytic Degradation of Methylene Blue Dye. Orient J Chem2025; 41(1). https://doi.org/10.13005/ojc/410605 |
[36]
.
Figure 7. Photocatalytic activity (a-c) and the degradation trend (d) of MB as a function of irradationtime of Aloevera leaf gel extract capped CdS NPs composed out of: (a) cadmiumchloride,(b) cadmium nitrate including (c) cadmium sulphate salts towards the decrease in absorbance.
Figure 7 (d) illustrates the degradation curves of aqueous MB solution exposed to visible radiation in existence of CdS NPs including degradation trend of pure MB dye without any catalyst.
Figure 7 makes it abundantly evident that after 180 min of photocatalytic investigation, no new peaks are seen and the parent absorption peaks have significantly decreased, indicating that the photo-degradation of MB has been assessed to be complete. The degradation of strong absorption peak is decresing gradually in case of
Figure 7 (a) and (b), where there is a sudden escalation in the maximum absorption in
Figure 7 (c) after 60 min in dark and followed by 30 min exposure to visible light. This indictes that the photocatalytic activity is more prominent in presence of light irradiation.
A sudden escalation in peak intensity or the maximum absorption curve in
Figure 7 (c) of a CdS NPs photocatalysts is typically may be due to an increase in the concentration of the catalyst or a change in the physical state of the NPs, such as agglomeration. It could also be due to an adsorption-desorption dynamics because an initial phase of a photo catalysis involves an adsorption process in the dark and an escalation after 60 min in dark and 30 min exposure in light might be observed if a significant amount of dye was initially adsorbed onto the CdS NP surface, and then some experimental change caused a sudden desorption of these molecules back into the solution, increasing the concentration of free dye in the measured volume.
The photocatalytic activity of Aloe vera leaves surfactant stabilized CdS NPs is assessed by an absorption spectrum of the RhB dye having absorption peak at around 552 nm over a period of 180 min is displayed in
Figure 8 (a-c). The paramount absorption peak wavelength shift happened to higher wavelength side after 60 min observation in dark for the Aloe vera leaves gel surfactant capped CdS NPs made from cadmium chloride along with the cadmium sulphate. It can be attributed to cadmium to sulfur precursor ratio employed as 1:2 which indicates more sulfur content and also higher pH value
| [37] | Hong L, Cheung T L, Rao N, Ouyang Q, Wang Y, Zeng S, Yang C B, Cuong D, Han P, Chong J, Liu L, Law W C and Yong K T: Millifluidic Synthesis of Cadmium Sulfide Nanoparticles and Their Application in Bioimaging, RSC Adv., 7 (2017) 36819–36832. https://doi.org/10.1039/C7RA05401G |
[37]
. Whereas for the Aloe vera assisted CdS NPs made out of cadmium nitrate shows the shifting of maximal absorption wavelength from 552 nm to a lower wavelength side be observed and depicted by
Figure 8 (b). The
Figure 8 (d) exhibits degradation trend of the RhB dye that is variation in concentration of the dye along with time for Aloe vera leaf extract assisted CdS NPs as well as pure RhB dye.
Figure 8. Photocatalytic activity (a-c) including the degradation trend (d) of RhB as a function of irradation time of Aloevera leaf gel stabilized CdS NPs prepared from: (a) cadmium chloride,(b) cadmium nitrate, (c) cadmium sulphate towards decrease in absorbance.
The photocatalytic effectiveness of CdS NPs' manufactured by the cadmium precursors like cadmium chloride, cadmium nitrate including cadmium sulphate disclose 39, 31 along with 39% against RhB dye, respectively. The shift of absorption wavelength from 552 nm to a lower wavelength side that is around 494 nm for the CdS NPs armed out of cadmium nitrate of RhB dye degradation depicts by
Figure 8(d) gives the clear evidence of the evolution of the complete de-ethylated RhB molecule.
3.7. PL Study of Aloe Vera Leaves Gel Extract Stabilized CdS NPs
The light emission of a material at a specific wavelength can be identified using photoluminescence which is a measurement of photo absorption in a direct band gap materials. The PL spectra are being carried out to explore the luminescence characteristic of the Aloe vera leaf gel extract assisted CdS NPs produced from cadmium chloride, cadmium nitrate and cadmium sulphate correspond to (a), (b) including (c) are displayed in PL spectra 9 (a-c). The PL spectrum is captured in the region of wavelength 200-800 nm.
PL graphs of Aloe vera stbilized CdS NPs in
Figure 9(a)-(c), exhibit a band edge luminescence that can be identified by a narrow strong sharp edge peak at around 492 nm. The PL peak at around 512 nm can be seen in
Figure 9(a) with medium intense broad band and that of small intense low broad band at around 512 nm in
Figure 9(b) and almost no peak at the same wavelength in
Figure 9(c).
Figure 9. Photoluminescence emission spectra of Aloe vera leaves gel extract encapsulated CdS NPs preparedfrom:(a)cadmiumchloride,(b)cadmiumnitrateincluding, (c)Cadmium sulphatesalts.
The low energy of the PL band indicates that the CdS excitonic dispersion is not what causes the PL maximum at about 512 nm. Additionally, with restricted CdS NPs, the PL excitonic peak is typically found at 410 nm
. Because the PL band energies being below or closely comparable to that of the band gap energy, the luminescence bands may be distinguished from transitions comprising donors, acceptors along with surface traps
. This band, as compared to that of bulk CdS, is almost certainly the outcome of recombination via deeper imperfection states
| [40] | Nesheva D, Raptis C, Levi Z, Popovic Z, Hinic I: Photoluminescence of CdSe Nanocrystals Embedded in a SiO2Thin Film Matrix. Journal of Luminescence, 82 (1999) 233–240. |
[40]
. Additionally, it implies that this shallow state donor-acceptor interaction in the CdS energy gap can lead to a small-energy range
| [41] | Chahboun A, Rolo A G, Filonovich S A, Gomes M J M: Factors Influencing the Passivation of CdS Quantum Dots Embedded in Silica Glass. Solar Energy Materials and Solar cells., 90 (2006), 1413–1419.
https://doi.org/10.1016/j.solmat.2005.10.006 |
[41]
. As a result, native defects like sulfur or cadmium vacancies are primarily responsible for the defect levels that contribute to low lying energy band edge PL
| [42] | Alipour A, Lakouraj M M, Tashakkorian H: Study of The Effect of Bandgap and Photoluminescence on Biological Properties of Polyaniline/CdS QD Nanocomposites Based on Natural Polymer., Nature Research - Scientific Reports., 11 (2021) (1-15). https://doi.org/10.1038/s41598-020-80038-1 |
[42]
. Various trapping modes on NPs may be accountable for the decline in PL peak intensity
| [43] | Alani R R, Ibrahim O A: Effect of Point Defects on the Structural and Optical Properties of CdS Nanoparticles Synthesized by Chemical Method, International Journal of Mechanical Engineering, 7 (2022) 5156-5165. |
[43]
which is illustrated in
Figure 9 (a) and (b).
3.8. Discussions
The CdS NPs are being synthesized successfully by applying various cadmium salts along with the natural surfactant Aloe vera leaves gel extract. The length of time needed for a reaction using distinct sources of cadmium and sulphur in various molecular quantities, in addition to different reaction allocations, stirring velocities and variable ratios of natural surfactant, is being estimated with suitable mole fractions of cadmium salts, weight percentages of natural extracts are employed in the synthesis process of CdS NPs. It is found that for 0.1 M or 0.05 M of cadmium salts including sodium sulfide of 0.1 M, 4 hrs reaction time and stirring speed of 900 rpm and temperature at 60°C giving the better outcome for the surfactant agent Aloe vera leaf gel extract.
Factors for synthesis used in the process of producing CdS NPs steadiness by Aloe vera leaf gel surfactant, cadmium chloride, cadmium nitrate including cadmium sulphate is expressed in
Table 2.
Table 2. Specifics of the synthesis parameters used to synthesize CdS NPs.
S.No. | Natural stabilizer | Cd Precursor | Mole fraction of Cd: S | Source of Sulfur | StirringTime(hrs) | pH | Extract wt.% inCd source |
1 | Aloe vera Gel extract | CdCl2 | 1:2 | Na2S | 4 | 11 | 40 |
Cd(NO3)2 | 1:2 | Na2S | 4 | 11 | 30 |
CdSO4 | 1:1 | Na2S | 4 | 6 | 30 |
Table 3 lists the average particle size of the as-prepared CdS NPs derived from SAXS, SEM, along with TEM investigations as well as the corresponding crystallite sizes from XRD investigations. Presence of additional peaks other than CdS which corresponds to sodium chloride is a limitation for the CdS NPs prepared from cadmium chloride precursor. It has been established that CdS NPs are generated with sizes less than 9 nm and all of these values are nearly identical to one another. Particle size usually decreases with increasing pH. The pH level is changed in the current investigation to produce tiny CdS NPs with a particle size of less than 9 nm.
The crystallite sizes calculated from XRD and average NP size calculated from TEM are in good agreement for the CdS NPs prepared from cadmium nitrate and cadmium sulphate precursors whereas discrepancy in calculated the crystallite size for CdS NPs obtained from XRD which is 8 nm and that calculated from TEM is 2.6 nm may be attributed to the presence of coarse-grained powder and cluster particles.
The surface of NPs significantly influences their physical also chemical characteristics. The PL phenomenon is notably influenced by the surfactants, necessitating meticulous measures to eliminate probable trap sites and attain elevated fluorescence quantum yields. In case of CdS, it is observed that two distinct forms of emission can occur, namely band edge emission corresponds to wavelength below 500 nm and the surface defect emission, which is attributed to the wavelength more than 500 nm. The active species involved in photocatalytic degradation of dyes are holes as well as super oxide radicles played a significant role
| [44] | SrinivasaGoud B., Suresh Y., Annapurna S., BhikshamaiahG., TarunBabu M., A. K. Singh: Photoluminescence and Photocatalytic studies of rice water and papaya fruit extract encapsulated cadmium sulfide nanoparticles, Journal of the Korean Ceramic Society, 60 (3) (2022) 1-20.
https://doi.org/10.1007/s43207-022-00253-6 |
[44]
.
Table 3. Average size of as synthesized CdS NPs determined from SAXS, SEM as well as TEM characterization studies along with their respective average crystallite sizes by XRD analysis.
S.No | Surfactant | Notation | Source of Cd | XRD(nm) | SEM(nm) | SAXS(nm) | TEM(nm) |
1 | Aloe vera GelExtract | A | CdCl2 | 8 | 2.10 | 2-4 | 2.61 |
B | Cd(NO3)2 | 3 | 2.16 | 5-12 | 2.51 |
C | CdSO4 | 3 | 1.60 | 3-9 | 2.62 |
Stoichiometric ratio of as prepared CdS NPs according to the natural stabilizers is illustrated in
Table 4.
Table 4. The stoichiometric ratio, band gap values and morphology of as prepared CdS NPs capped with Aloe vera leaf gel stabilizer.
S.No. | Natural Stabilizer | Cd Source | Atomic wt.% Cd: S | SCR; Cd: S | Band gap (eV) Max-Min | Morphology of CdS NPs |
5 | Aloe vera leaf Gel extract | CdCl2 | 7.89:10.34 | 1:1.31 | 5.04 | Both spherical, facet shape |
Cd(NO3)2 | 1.43:1.97 | 1:1.37 | 5.18 | Both spherical, irregular shape |
CdSO4 | 5.83:10.95 | 1:1.87 | 5.09-3.58 | Spherical and irregular shape |
The emission of blue light is ascribed to electron-hole pair disintegration through radiative action. The PL spectrum of CdS NPs, which are capped with surfactants, exhibited comparable characteristics to those of CdS NPs without any capping agent, however with slightly higher fluorescence intensity and blue shift of high intensity peaks. It can be inferred that the presence of natural surfactants has the potential to enhance the fluorescence of uncapped CdS. The PL emission peaks traced at 491, 492 nm which are being better acceptance to that of reported value and are due to sulphur vacancy to valence band
| [45] | Chaure S, Chaure N B, Pandey R K, Ray A K: Stoichiometric Effects on Optical Properties of Cadmium Sulphide Quantum Dots, IET Circuits Devices Syst., 1 (2007) 215–219.
https://doi.org/10.1049/iet-cds:20070048 |
[45]
transition of electron represented by sulphur vacancy
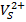
→V
bvalence band edge. The luminescent emission peak located at 493 nm almost coincides with the reported value and peak position at 499 nm little deviates with 5 nm to that of the reported value and these peaks are due to an excitonic emission which comes under near band edge emission.
The PL peaks located at 509 deviates 3 nm from reported value, 511 and 512 are because of electron transitions between donor to acceptor level, which can be demonstrated as interstitial cadmium
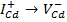
acceptor levels
| [46] | Berry A K, Amirtharaj P M, Jing-Tong Du, Boone J L, Martin D D: Photoluminescence and Raman Studies of CdS Films Grown by Metal-Organic Chemical Vapour Deposition on Si{111} Substrates, Thin Solid Films, 219 (1992) 153-156.
https://doi.org/10.1016/0040-6090(92)90736-U |
[46]
. Transition due to interstitial sulphur sites gives raise the peak at 514 nm
| [9] | Singh V, Chauhan P: Structural and Optical Characterization of CdS Nanoparticles Prepared by Chemical Precipitation Method. Journal of Physics and Chemistry of Solids, 70 (2009) 1074–1079. https://doi.org/10.1016/j.jpcs.2009.05.024 |
[9]
which can be illustrated as interstitial sulphur
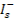
→ C
bconduction band edge.
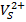 →Vbvalence band edge. The luminescent emission peak located at 493 nm almost coincides with the reported value and peak position at 499 nm little deviates with 5 nm to that of the reported value and these peaks are due to an excitonic emission which comes under near band edge emission.
→Vbvalence band edge. The luminescent emission peak located at 493 nm almost coincides with the reported value and peak position at 499 nm little deviates with 5 nm to that of the reported value and these peaks are due to an excitonic emission which comes under near band edge emission. 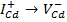 acceptor levels
acceptor levels 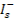 → Cbconduction band edge.
→ Cbconduction band edge.